 |
| Source |
CO2 Enthalpy of formation = -393kJ/mol*
CO Enthalpy of formation = -110kJ/mol (28% as much per-mol or 56% as much on a per unit of carbon)
SO2 Enthalpy of formation = -297kJ/mol
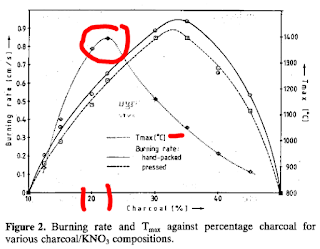
This data suggests that if one can control the burn-rate through granule size, then the highest pressures for sulphur-free blackpowder will be achieved within the range of 17%-to-20% charcoal that is ground to a mean particle size of 15-to-35 microns and then aggressively mixed with enough 75% water-25% alcohol before pucking to ensure that every charcoal granule is intimately "wetted" with KNO3.
Since burn-rates for zero-sulphur blackpowders tends to be slower than with sulphur, one size smaller granules (fffg instead of ffg) is worth investigating.
Some people like to state ratios with the most plentiful component set at 100. A traditional 75%-15%-10% (KNO3-C-S) mix would be expressed as 100-20-13. A black powder with a mix of 81%-19%-0% (KNO3-C-S) would be expressed as 100-23-0. This way of noting the ratios is convenient for measuring. If you measure out 200 grams of KNO3 then it is trivial to calculate the amounts of the other ingredients.
* mol or mole is approximately 60,221,415,000,000,000,000,000 molecules.

A lot of inadvertent KABOOM's occurred over the centuries as this knowledge about black powder was acquired. If one wishes to make and use black powder one must be very careful to not add to those statistics. The stuff does not take kindly to mistakes.
ReplyDeleteThe experimentation that likely occurred over the years to get to this point should be respected.
ReplyDeleteCapt. Kirk whipped together gunpowder lickety-split to kill the Gorn in the Arena...
ReplyDelete